orange book pharmacy definition
The electronic availability of the Orange Book brings this valuable tool to the web for. Type The group or category of approved drugs.

3 Uses For Historical Versions Of The Fda Orange Book Drugpatentwatch Make Better Decisions
Book was published in October 1980 with orange cover and thus the name orange book.

. Provides a listing of drugs approved as. Page 1 Definition It is the publication of Approved Drug Products with. Governments now obsolete standards document Trusted Computer System Evaluation Criteria DOD standard 520028-STD December 1985 which characterize.
The FDAs Orange Book or Approved Drug Products with Therapeutic Equivalence Evaluations lists products with their corresponding therapeutic equivalents. As of May 2020. The Orange Book has long been a reliable resource for information about FDA-approved drugs.
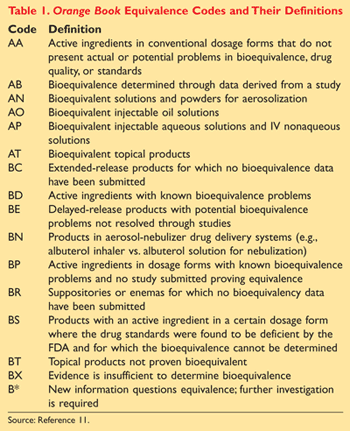
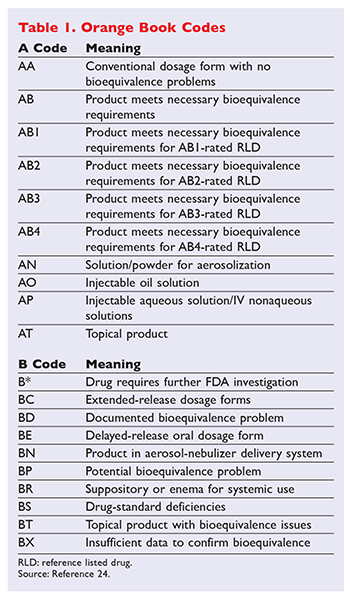
The orange book is published annually and the 2015 edition is 35th edition of orange. In terms of the Orange Book explain the first letter of the 2-letter coding system for the therapeutic equivalence of multi-source drug products Second letter is FYI First letter is A. Page 2 Contents of.
Sumanta Mondal_MPharm 1 Sem. In the electronic Orange Book a reference standard is identified by RS in the RS column. Approved Drug Products with Therapeutic Equivalence Evaluations.
Search approved drug products by active. Rucha Pathak Roll No. It is prepared by The Orange Book Staff.
Sumanta Mondal_MPhar m 1 th Sem. The orange book is published annually and the 2015 edition is 35th. CDR Kendra Stewart RPh PharmD.
Definition It is the. Approved Drug Products with Therapeutic Equivalence Evaluations. Originally this book was published in October 1980 with orange cover and thus the name orange book.
The orange book is a list of generic drugs approved by FDA. The Orange Book is an FDA publication mandated under section 505j7A of the Federal Food Drug Cosmetic Act FDC Act which. _ GITAM Institute of Pharmacy.
Preface to Approved Drug Products with Therapeutic Equivalence Evaluations Orange Book provides info on how the book came to be relevant terms and codes user. Office of Generic Drugs Policy Center for Drug Evaluation Research US. On March 23 2020 FDA removed from the Orange Book the listings for biological products that have been.
Food and Drug Administration. The orange book is published annually and the 2015 edition is 35th edition of orange. On March 23 2020 FDA removed from the Orange Book the listings for biological products that have been.
_ GITAM Institute of Pharmacy. Multicultural Pharmacy Scholars Program. THE ORANGE BOOK thLecture Notes_Dr.
Formally known as Approved Drug Products with Therapeutic. 2 approved over-the-counter OTC drug products for those drugs that. Objectives What is a Generic Drug.
Search the Orange Book Database. Format is RX OTC DISCN. For more information on the Orange Book including its history see the Orange Book Preface.

3 Uses For Historical Versions Of The Fda Orange Book Drugpatentwatch Make Better Decisions
Optimizing Generic Medication Use

Orange Book And Its Applications Legal Advantage
Insights Into Effective Generic Substitution

Otc Introduction And Definitions Ppt Video Online Download

Medical Prescription Wikipedia

Approved Drug Products With Therapeutic Equivalence Evaluations T H E O R A N G E B O O K Ppt Download

The Introduction Of An Orange Book

Drug Channels Defining Specialty Pharmacy

Drug Pricing And Pharmaceutical Patenting Practices Everycrsreport Com

Pharma Treasures Terminology And Definitions Related To Pharmaceutical Industry Glossary Of Pharma Related Terms Pharmaceutical Industry Terms Definitions
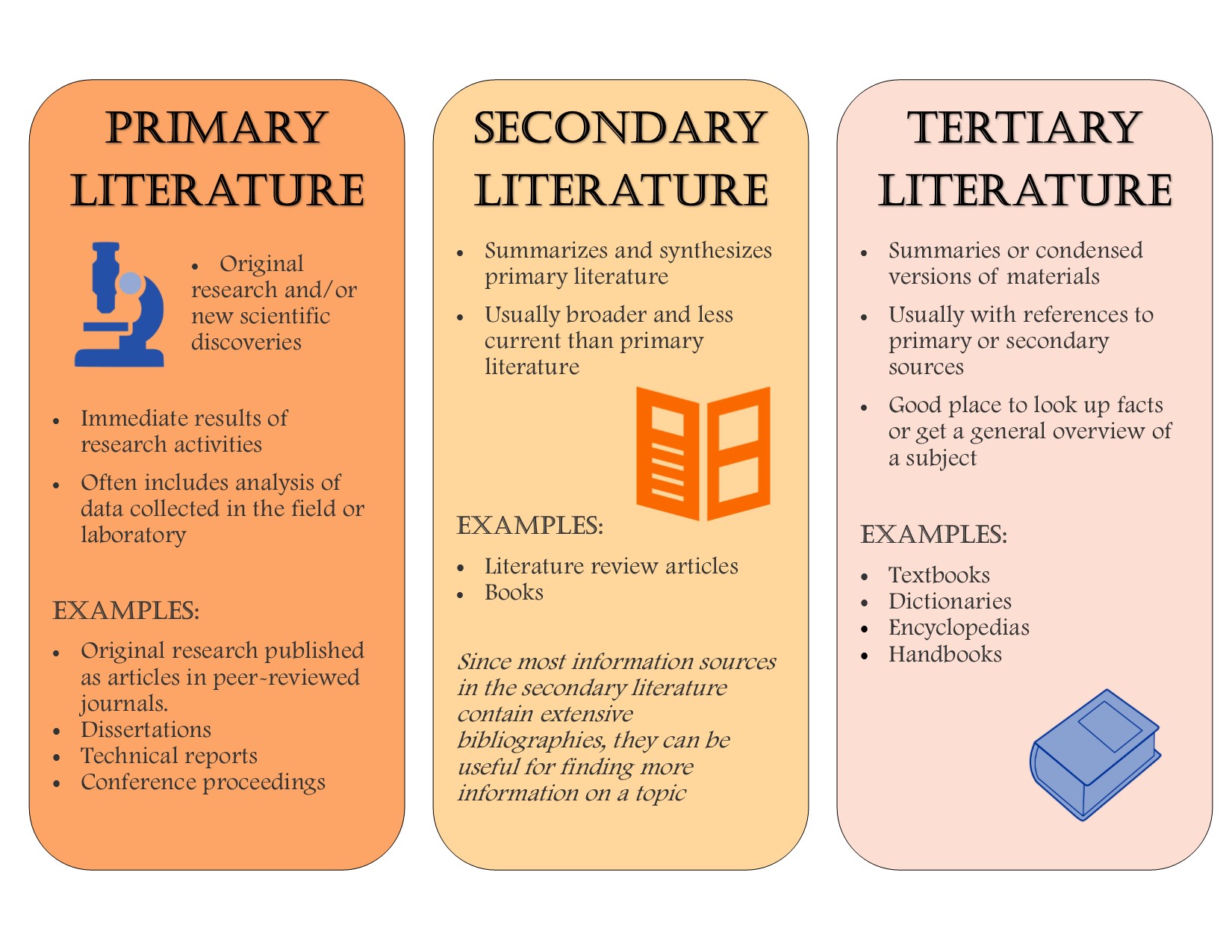
Primary Secondary And Tertiary Literature In The Sciences Pharmacy Research Resources Libguides At Ohio Northern University

Understanding Authorized Generics A Review Of The Published Clinical Data Alderfer 2021 Journal Of Clinical Pharmacy And Therapeutics Wiley Online Library

Orange Book Editions Content Approved Drug Products With Therapeutic Equivalence Evaluations Youtube
What Is A Drug Formulary Definitions Tiers Costs And More Goodrx

What S In A Name Drug Nomenclature And Medicinal Chemistry Trends Using Inn Publications Journal Of Medicinal Chemistry

/doctor-826e0c116cd549d98e327f1184c622d9.jpg)